INTRODUCTION: Myeloid neoplasms with mutated TP53 (MN-TP53) represent a unique category in the 2022 International Consensus Classification (ICC) of Myeloid Neoplasms. This entity has an exceedingly poor prognosis with the available standard therapies, and clinical trials with novel drugs show limited benefits. Recently, it has been shown that TP53 multi-hit alterations may confer even worse prognosis, compared to the monoallelic status, in myelodysplastic syndromes. However, multi-hit status has not been analysed in all MN-TP53 subtypes. Elucidating this difference and its association with responses to treatment becomes essential from a clinical perspective. Therefore, our aim was to analyse whether the allelic status of TP53 in our cohort has an impact on therapeutic outcomes.
METHODS: We retrospectively collected data from four institutions, between September 2011 and February 2023. Inclusion criteria were: 1) patients diagnosed with MN-TP53; 2) available information to categorize them into multi-hit or monoallelic group according to ICC 2022; and 3) eligible for treatment. The primary endpoint was to describe the outcomes, in terms of overall response rate (ORR) and overall survival (OS), of MN-TP53 according to TP53 status (multi-hit vs monoallelic) and treatment.
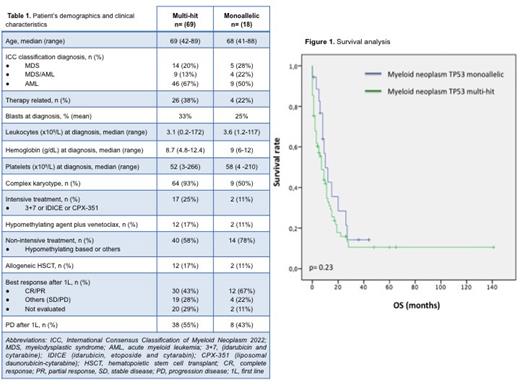
RESULTS: A total of 105 patients diagnosed with MN-TP53 were identified. Eighty-seven were eligible for treatment and included in the analysis. Sixty-nine patients were identified as multi-hit, and 18 as monoallelic. Table 1 summarises patient's demographics and clinical characteristics. The median OS of the entire cohort was 7 months (range 0-141); while it was 6 months (0-141) for the multi-hit group and 8.5 months (0-44) for the monoallelic group; (p=0.23) (Figure 1). In both groups, most of the patients received non-intensive treatment as first line (1L) (40 patients [58%] in multi-hit group vs 14 [78%] in monoallelic), followed by intensive treatment (17 patients [25%] in multi-hit group vs 2 patients [11%] in monoallelic) and hypomethylating agent plus venetoclax (12 [17%] in multi-hit vs 2 [11%] in monoallelic). The overall response rate (ORR) was 43% for patients with multi-hit TP53 status and 67% for those with monoallelic status (p=0.38) . Correspondingly, 29% and 11% were not evaluated after 1L due to death or poor general condition. Progression after 1L of treatment was seen in 55% of patients with multi-hit TP53 status and in 43% of cases with monoallelic status (p=0.11). The subgroup analysis of the multi-hit cohort revealed no significant difference in response between intensive and non-intensive treatment (p=0.09). Nevertheless, there is a trend in favour of non-intensive treatment, including hypomethylating agents plus venetoclax (p=0.18). Allogeneic hematopoietic stem cell transplant was performed in 12 patients (17%) in the multi-hit group and in 2 patients (11%) in the monoallelic group.
CONCLUSION: In this study, no difference in terms of OS was evidenced when comparing patients according to the type of TP53 status (multi-hit vs monoallelic). However, a trend to higher ORR and lower rate of progression was observed in the monoallelic group after 1L, which should be confirmed in further analysis. Concerning the TP53 multi-hit subgroup, we have not found any benefit on using intensive treatment in terms of ORR compared to non-intensive approach.
Disclosures
Tormo:Astellas: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; MSD: Honoraria; BMS: Honoraria. Tazon:Bristol Myer Squibb: Honoraria. Jerez:Novartis: Consultancy; Astrazeneca: Research Funding; GILEAD: Research Funding; BMS: Consultancy. Salamero:BMS: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal